Research Focus
Cell programming and neural fate determination
In vitro differentiation of human pluripotent stem cells (PSC) provides a fascinating experimental window into early neural cell type specification. The Brüstle Team uses this approach to study fundamental mechanisms underlying stemness and differentiation and to generate intermediate neural stem and progenitor cell populations for a broad variety of neuronal and glial cell types. 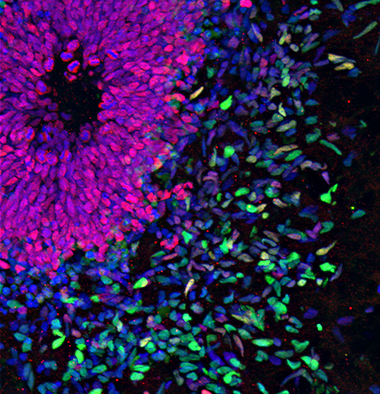 Methodology includes extrinsic factor-driven differentiation and patterning as well as transcription factor-based forward programming.
Methodology includes extrinsic factor-driven differentiation and patterning as well as transcription factor-based forward programming.
Self-organization of PSC-derived neural cells into spheroids and organoids is employed to experimentally address brain architecture formation and cell-cell interactions in a neural tissue-like environment.
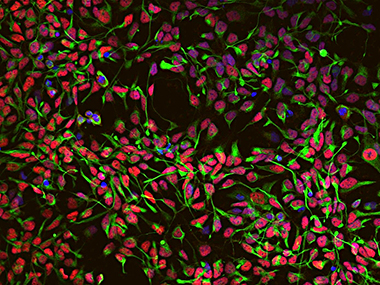 IRN scientists further use controlled expression of transcription factors and small molecule-based modulation of cell signaling pathways to implement new routes for direct cell fate conversion, including the generation of induced neurons (iN) and induced neural stem cells (iNSC).
IRN scientists further use controlled expression of transcription factors and small molecule-based modulation of cell signaling pathways to implement new routes for direct cell fate conversion, including the generation of induced neurons (iN) and induced neural stem cells (iNSC).
Stem cell-based disease modeling
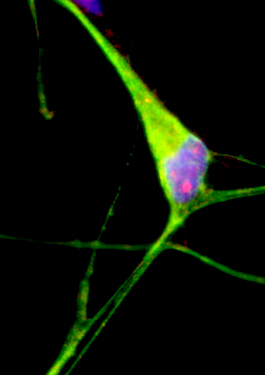 Reprogramming into iPS cells and direct conversion of blood or skin fibroblasts into neural cells provide exciting routes for generating patient-specific neurons and glia for disease modeling and drug discovery. Combined with gene editing these approaches enable a sensitive read-out of disease-associated pathophenotypes. The Brüstle team uses this methodology for modeling neurodegenerative and psychiatric disease and to establish assay systems for compound validation and drug discovery. In this context, classic 2D cultures are increasingly complemented by 3D matrix and organoid cultures to address disease-associated defects in brain architecture formation and extracellular pathologies.
Reprogramming into iPS cells and direct conversion of blood or skin fibroblasts into neural cells provide exciting routes for generating patient-specific neurons and glia for disease modeling and drug discovery. Combined with gene editing these approaches enable a sensitive read-out of disease-associated pathophenotypes. The Brüstle team uses this methodology for modeling neurodegenerative and psychiatric disease and to establish assay systems for compound validation and drug discovery. In this context, classic 2D cultures are increasingly complemented by 3D matrix and organoid cultures to address disease-associated defects in brain architecture formation and extracellular pathologies.
Neural transplantation & regeneration
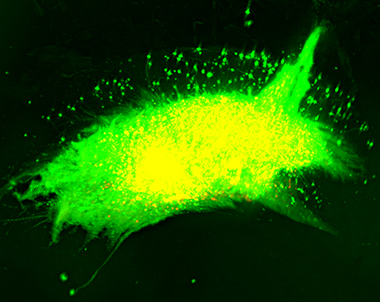 Experimental transplantation of neural cells into the rodent brain provides a highly informative approach for assessing differentiation and function of in vitro generated human neural cells in a real life scenario. Having a strong tradition in neurotransplantation, the Brüstle team uses this approach to study patient-derived and control cells in vivo, to assess the efficacy of stem cell-based therapies in a preclinical setting and to devise new routes for enhancing synaptic integration of transplanted human neurons.
Experimental transplantation of neural cells into the rodent brain provides a highly informative approach for assessing differentiation and function of in vitro generated human neural cells in a real life scenario. Having a strong tradition in neurotransplantation, the Brüstle team uses this approach to study patient-derived and control cells in vivo, to assess the efficacy of stem cell-based therapies in a preclinical setting and to devise new routes for enhancing synaptic integration of transplanted human neurons.
 Institute of Reconstructive Neurobiology
Institute of Reconstructive Neurobiology