Research Focus
Background
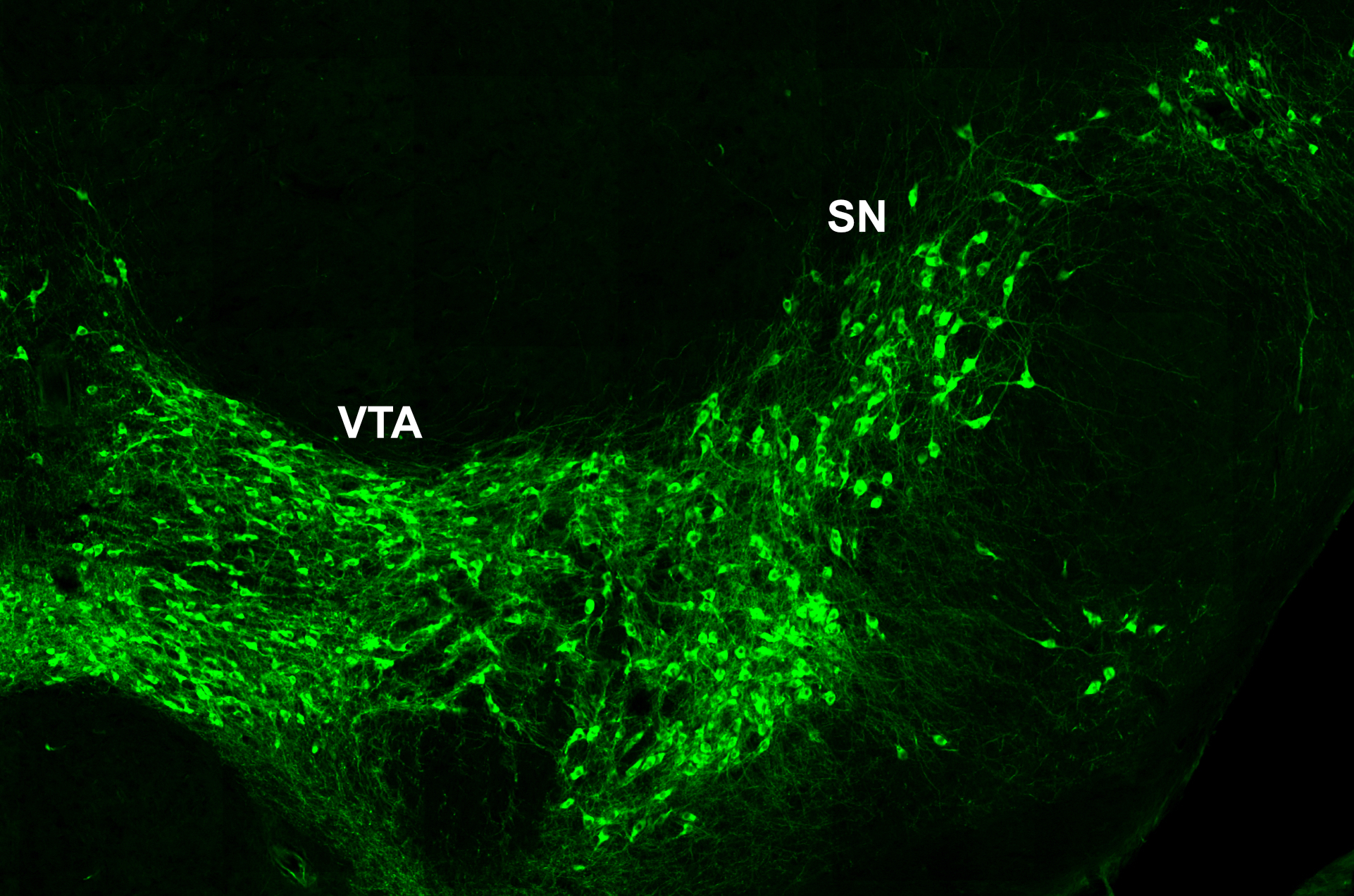 Our research focuses on the development of the midbrain dopaminergic system. Midbrain dopaminergic (mDA) neurons modulate cognitive processes, regulate voluntary movement and reward behavior. Degeneration of a subset of mDA neurons in the substantia nigra (SN) underlies the motor deficits in Parkinson’s disease, while altered dopamine transmission is implicated in neuropsychiatric disorders including depression, schizophrenia and substance abuse. In the last couple of years, anatomically and physiologically discrete dopaminergic circuits and their impact on distinct aspects of behavior have been dissected in increasing detail, propelled by the rapid progress in opto- and chemogenetic techniques and viral tracing systems. Thus, it has become clear that the dopaminergic system is composed of diverse populations of mDA neurons and that this diversity is crucial for the various functional outputs of the dopaminergic system.
Our research focuses on the development of the midbrain dopaminergic system. Midbrain dopaminergic (mDA) neurons modulate cognitive processes, regulate voluntary movement and reward behavior. Degeneration of a subset of mDA neurons in the substantia nigra (SN) underlies the motor deficits in Parkinson’s disease, while altered dopamine transmission is implicated in neuropsychiatric disorders including depression, schizophrenia and substance abuse. In the last couple of years, anatomically and physiologically discrete dopaminergic circuits and their impact on distinct aspects of behavior have been dissected in increasing detail, propelled by the rapid progress in opto- and chemogenetic techniques and viral tracing systems. Thus, it has become clear that the dopaminergic system is composed of diverse populations of mDA neurons and that this diversity is crucial for the various functional outputs of the dopaminergic system.
Development of diversity in the dopaminergic system
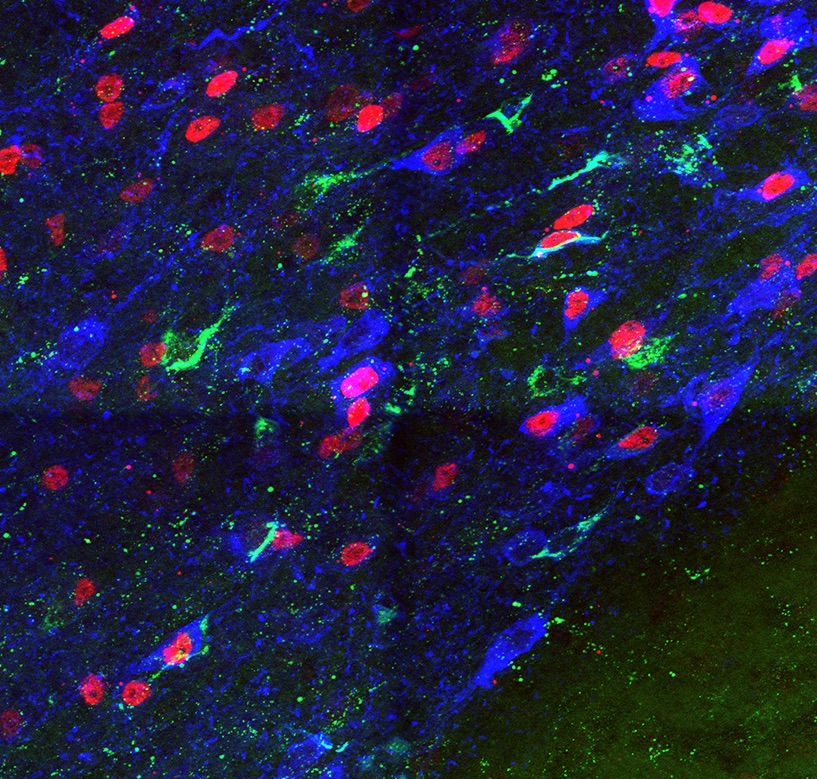 A major goal of our research effort is to unravel the mechanisms that underlie the generation of diversity in the dopaminergic system. To investigate how these developmental processes determine the connectivity and function of mDA neuronal subtypes in the adult brain, we use a combination of developmental genetic approaches, genetic tracing tools, electrophysiology and optogenetic methods. Given that it is still unclear why specific subgroups of mDA neurons are susceptible to neurodegeneration in Parkinson’s disease and that aberrant dopamine regulation is associated with several neuropsychiatric disorders, a comprehensive knowledge of mDA neuronal development is a prerequisite for understanding the etiology of these diseases and will contribute to the development of novel therapeutic strategies.
A major goal of our research effort is to unravel the mechanisms that underlie the generation of diversity in the dopaminergic system. To investigate how these developmental processes determine the connectivity and function of mDA neuronal subtypes in the adult brain, we use a combination of developmental genetic approaches, genetic tracing tools, electrophysiology and optogenetic methods. Given that it is still unclear why specific subgroups of mDA neurons are susceptible to neurodegeneration in Parkinson’s disease and that aberrant dopamine regulation is associated with several neuropsychiatric disorders, a comprehensive knowledge of mDA neuronal development is a prerequisite for understanding the etiology of these diseases and will contribute to the development of novel therapeutic strategies.
Mechanisms underlying the formation of the substantia nigra (SN) and ventral tegmental area (VTA)
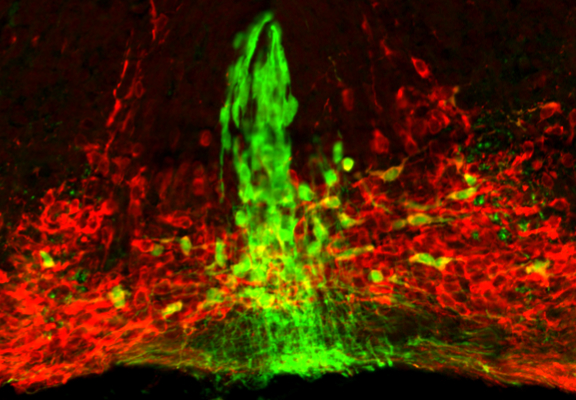 The exact anatomical organization of mDA neurons in the ventral midbrain likely contributes to the formation of functional circuits, since the precise positioning of mDA cell bodies in the ventral midbrain ensures that afferent and efferent projections encounter the proper cues guiding them to appropriate targets. Newly differentiated SN- and VTA-mDA neurons undergo radial migration into the mantle layer of the developing ventral midbrain. Subsequently, only SN-mDA neurons migrate laterally. This migration pattern suggests that VTA- and SN-mDA neurons have specific molecular machineries to respond to environmental cues directing their distinct migration. A prerequisite to unravel how molecular mechanism regulate these specific cell behaviors is an understanding of the dynamic cellular changes that occur in migrating neurons. We are investigating the migratory behavior of mDA subpopulations as well as the underlying cellular and molecular mechanisms that regulate their migration using genetic approaches and time-lapse imaging.
The exact anatomical organization of mDA neurons in the ventral midbrain likely contributes to the formation of functional circuits, since the precise positioning of mDA cell bodies in the ventral midbrain ensures that afferent and efferent projections encounter the proper cues guiding them to appropriate targets. Newly differentiated SN- and VTA-mDA neurons undergo radial migration into the mantle layer of the developing ventral midbrain. Subsequently, only SN-mDA neurons migrate laterally. This migration pattern suggests that VTA- and SN-mDA neurons have specific molecular machineries to respond to environmental cues directing their distinct migration. A prerequisite to unravel how molecular mechanism regulate these specific cell behaviors is an understanding of the dynamic cellular changes that occur in migrating neurons. We are investigating the migratory behavior of mDA subpopulations as well as the underlying cellular and molecular mechanisms that regulate their migration using genetic approaches and time-lapse imaging.
Modulatory inputs and the maturation of the prefrontal cortex
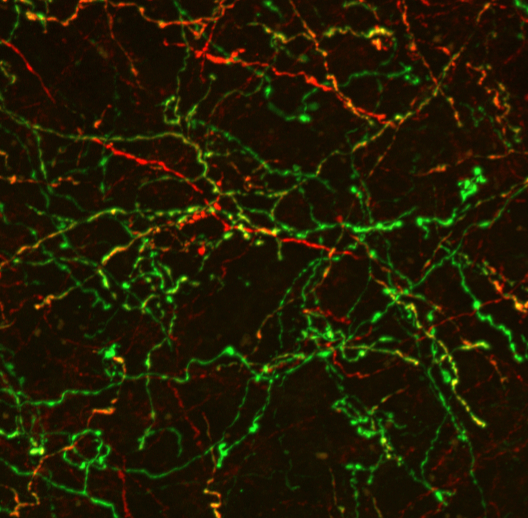 An additional goal of our research efforts is to investigate how modulatory inputs influence the development and maturation of the prefrontal cortex (PFC). The PFC controls key cognitive functions including executive decision making, attention and social interactions. Neuropsychiatric diseases such as schizophrenia and some of the psychopathological symptoms in neurodevelopmental disorders are associated with PFC dysfunction. Local circuits in the PFC as well as modulatory inputs mature over a protracted time period (up to early adulthood), making the PFC particularly vulnerable to aberrant development. We are investigating how the absence of modulatory inputs alters PFC circuit maturation and function. In addition, we are exploring how modulatory inputs into the PFC are altered in neurodevelopmental disorders and whether these potential alterations aggravate or ameliorate the functional alterations in the PFC in these disorders. We expect that these studies will provide important new insights in the role of modulatory innervation in setting up the basic circuitry of the PFC and will shed light on how modulatory inputs contribute to aberrant PFC function in neurodevelopmental and neuropsychiatric disorders.
An additional goal of our research efforts is to investigate how modulatory inputs influence the development and maturation of the prefrontal cortex (PFC). The PFC controls key cognitive functions including executive decision making, attention and social interactions. Neuropsychiatric diseases such as schizophrenia and some of the psychopathological symptoms in neurodevelopmental disorders are associated with PFC dysfunction. Local circuits in the PFC as well as modulatory inputs mature over a protracted time period (up to early adulthood), making the PFC particularly vulnerable to aberrant development. We are investigating how the absence of modulatory inputs alters PFC circuit maturation and function. In addition, we are exploring how modulatory inputs into the PFC are altered in neurodevelopmental disorders and whether these potential alterations aggravate or ameliorate the functional alterations in the PFC in these disorders. We expect that these studies will provide important new insights in the role of modulatory innervation in setting up the basic circuitry of the PFC and will shed light on how modulatory inputs contribute to aberrant PFC function in neurodevelopmental and neuropsychiatric disorders.
Methods and Techniques of the Neurodevelopmental Genetics group:
Mouse Models: Transgenes, conditional gene inactivation, mosaic gene inactivation, genetic inducible fate mapping.
Histology: Paraffin- and Cryosectioning, RNA in situ hybridization, Immunohistochemistry.
Molecular biology: PCR, RNA and DNA analysis, protein analysis, gene cloning and sequencing.
Cell culture: Brain explant cultures, primry cell cultures.
Microscopy: Fluorescence microscopy, fluorescence live imaging.
Electrophysiology: patch-clamp and optogenetics in brain slices.
 Institute of Reconstructive Neurobiology
Institute of Reconstructive Neurobiology